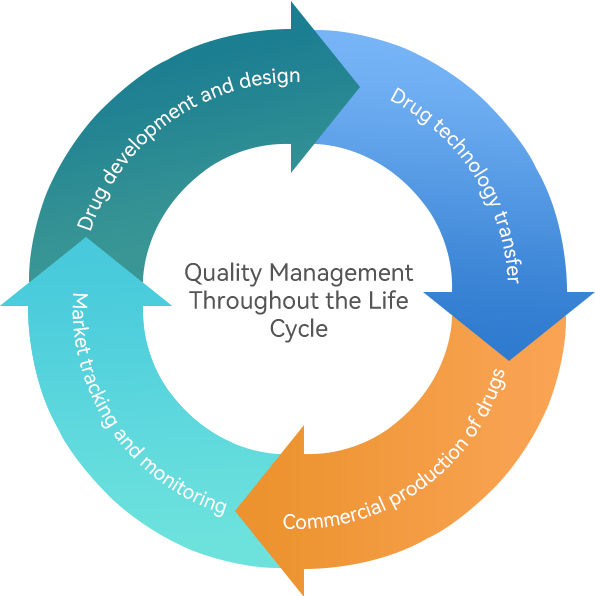
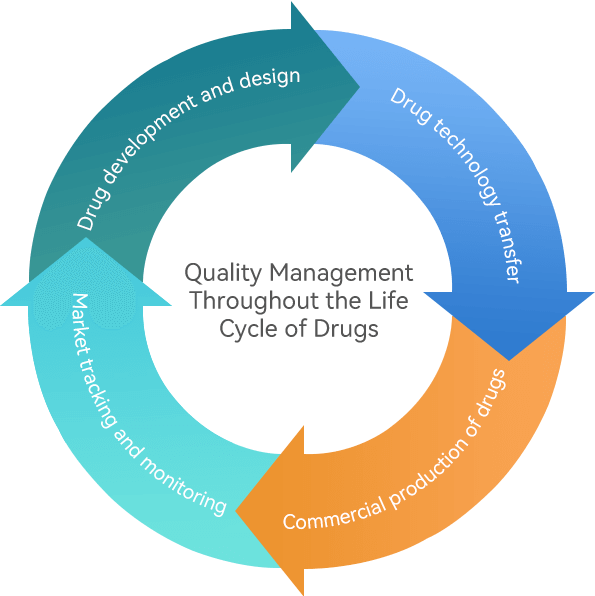
Our company adheres to the quality control concept of always maintaining consistency with the world's leading product access level, designing and constructing production lines according to international advanced standards, and configuring intelligent, digital production and testing equipment. Based on the full lifecycle quality control system implemented in drug research and development, drug production, quality control, product release, storage, transportation, and post market monitoring, we comprehensively guarantee product quality and safety through a strict quality testing and risk monitoring system, safeguarding patients’lives and health.
We actively follow the internationalization strategy, and have sold key formulations and APIs to Europe, America, Japan and other countries or regions; all sites have gained the ISO9001 quality management system certification; and all products have obtained the official GMP certification in China.